
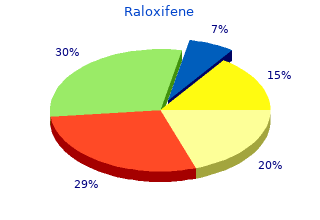
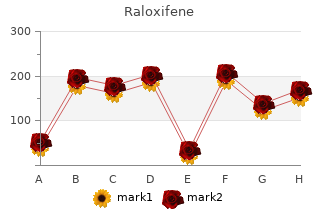
2018, Cogswell Polytechnical College, Pakwan's review: "Raloxifene 60 mg. Only $0.86 per pill. Effective Raloxifene OTC.".
Monitoring during oral sedation This involves alert clinical monitoring and at least the use of a pulse oximeter raloxifene 60mg with visa womens health specialists murfreesboro tn. The technique is unique as the operator is able to titrate the gas against each individual patient cheap raloxifene 60 mg visa pregnancy 6 months. That is to say, the operator increases the concentration to the patient, observes the effect, and as appropriate, increases (or sometimes decreases) the concentration to obtain optimum sedation in each individual patient. The administration of low-to-moderate concentrations of nitrous oxide in oxygen to patients who remain conscious. The precise concentration of nitrous oxide is carefully titrated to the needs of each individual patient. As the nitrous oxide begins to exert its pharmacological effects, the patient is subjected to a steady flow of reassuring and semi-hypnotic suggestion. This means that it is not possible to administer 100% nitrous oxide either accidentally or deliberately (the cut- off point is usually 70%). This is an important and critical clinical safety feature that is essential for the operator/sedationist. In addition to the machine head that controls the delivery of gases, it is also necessary to have a suitable scavenging system, and an assembly for the gas cylinders, either a mobile stand (Fig. The actual percentage of gases being delivered is monitored by observing the flow meters for oxygen and nitrous oxide, respectively (Fig. When the patient breathes out the reservoir bag gets larger as it fills with the mixture of gases emanating from the machine. Wait 60 s, above this level the operator should exercise more caution and consider whether further increments should be only 5%. With experience, operators will be able to judge whether further increments are needed. To bring about recovery turn the mixture dial to 100% oxygen and oxygenate the patient for 2 min before removing the nasal mask. The patient should breathe ambient air for a further 5 min before leaving the dental chair. The patient should be allowed to recover for a total period of 15 min before leaving. The above method of administration is the basic technique that is required in the early stages of clinical experience for any operator. This method ensures that the changes experienced by the patient do not occur so quickly that the patient is unable to cope. The initial time intervals of 60 s are used because clinical experience shows that shorter intervals between increments can lead to too rapid an induction and over- dosage. By careful attention to signs and symptoms experienced by the patient the dentist will soon be able to decide whether the patient is ready for treatment. The very rapid uptake and elimination of nitrous oxide requires the operator to be acutely vigilant so that the patient does not become sedated too rapidly. If the patient tends to communicate less and less, and is allowing the mouth to close, then these are signs that the patient is becoming too deeply sedated. The concentration of nitrous oxide should be reduced by 10 or 15% to prevent the patient moving into a state of total analgesia.
Examples of evaluative tools include the use and verification of pathophysiological and/or descriptive biomarkers for patient selection for clinical trials and/or use as surrogate endpoints generic 60 mg raloxifene otc womens health 2011. Included among those are Genentech’s trastuzumab (Herceptin) discount raloxifene 60mg mastercard women's health center shelton ct, which requires that patients be tested for particular genetic characteristics and the results be considered before the drug is administered. It analyzes the activities of 21 genes in a sample of breast tumor and then computes a score that is said to be predictive of whether a patient’s cancer will recur and whether she would benefit from chemotherapy. While there are only a few such complex tests on the market now, their number is expected to grow. Therefore, the agency needs to look at the data on which these tests are developed. Some might have to come off the market until the developer can provide enough data for approval. Government agencies have been criticized for not doing more to clamp down on questionable genetic tests that are being sold directly to consumers. Three components are needed to ensure the safety and quality of genetic tests: (1) the laboratories that conduct the tests must have quality control and personnel stan- dards in place to prevent mistakes; (2) the tests themselves must be valid and reli- able – i. Once these mechanisms are in place, uses and outcomes also must be evaluated over time in order to pinpoint any problems that may require attention, particularly as new tests enter wider use. However, the requirement could also discourage the development of diagnostics by raising the costs of introducing them. The requirement could discourage gradual improvements of tests because each change in a test might require a new regulatory submission. The process for reviewing such tests is “contingent on the intended use of the device” therefore, design of studies and data sets required will be influenced Universal Free E-Book Store 674 22 Regulatory Aspects of Personalized Medicine by a particular use. In this instance, a test for the prognosis of breast cancer would require different data than a test used to diagnose the disease. The ultimate goal of the project is to guide the choice of targeted therapy so that patients receive the most effective treatments. The development of therapeutic products that depend on the use of a diagnostic test to meet their labeled safety and effectiveness claims has become more common. For example, if a therapeutic product is only safe and effective in a patient subpopulation identi- fied by a diagnostic test, the Indications and Usage section of the labeling must define the patient subpopulation. Likewise, if a diagnostic test is essential for moni- toring beneficial or adverse effects, the Warnings and Precaution section must iden- tify the type of test. When appropriate, the labeling can name a class of therapeu- tic products, rather than specific products within the class. In addition, it supports the evaluation of qualitative results for a specific clinical analyte, including: • Preparation of control transcripts • Design of primers and amplicons • Quality control • Use in final experimental or clinical test application • Analysis and interpretation of data obtained This document is intended to help ensure comparable within-platform assay per- formance to enable comparisons of gene expression results. The protocols will enable research and clinical laboratories, regulatory agencies, accrediting agencies, reference laboratories, as well as test, microarray, and reagent manufacturers to assess the performance of these expression assays. Regulation of Direct-to-Consumer Genetic Testing Various states are beginning to tackle the problem of uncontrolled personal genetic services. In 2008, New York State, warned 23 companies that they must have per- mits to offer their services to New Yorkers. New York’s warning letter was a blow not only to new companies such as Navigenics (now acquired by Life Technologies) and 23andMe that entered into the field of consumer genomics in 2007, but also to Universal Free E-Book Store Regulation of Direct-to-Consumer Genetic Testing 677 technology suppliers Affymetrix and Illumina, which make the tools the testing companies use. In 2008, Department of Health of the State of California, in an effort to prevent consumer genetic testing companies from offering their services to the state’s residents, sent letters to 13 firms saying they are violating state law. One offense that genetic testing companies could commit would be to sell their products to California citizens over the Internet without the request or counsel of a physician. Another problem is that the companies’ tests have not been validated for accuracy or for clinical utility, which is required under California law. The Genetic Alliance, a nonprofit health advo- cacy organization committed to transforming health through genetics, has suggested that informed decisions must be made on the basis of analytic and clinical validity, clinical utility, and individual usefulness, as well as an understanding of oversight, regulation, and reimbursement (Zonno and Terry 2009).
SHARE THE DANA LANDSCAPING PAGE