
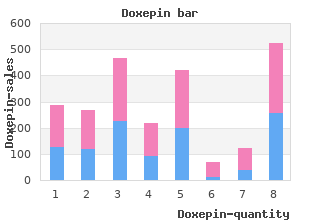
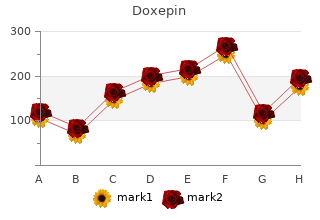
2018, Menlo College, Alima's review: "Doxepin 75 mg, 25 mg, 10 mg. Only $0.23 per pill. Cheap online Doxepin no RX.".
The half-life of cyclosporine is 7 to 19 hours in children and 19 to 40 hours in adults discount 75mg doxepin with visa anxiety symptoms muscle twitches. Metabolites are excreted primarily through the bile into feces; approximately 6% of cyclosporine is eliminated in the urine purchase doxepin 25mg anxiety 6 months after quitting smoking, with 0. Sandim- mune® oral solution may be diluted with milk, chocolate milk, or orange juice. Mix cyclosporine in a glass con- tainer and rinse the container with more diluent to ensure that the total dose is taken. However, many transplant cent- ers administer cyclosporine as divided doses (2–3 doses/day) or as a 24-hour continuous infusion. Patients should be under continuous observation for at least the first 30 minutes of the infusion, and should be monitored frequently thereafter. Pediatric Heart Transplantation 195 Monitoring Parameters Blood/serum drug concentration (trough), renal and hepatic function, serum electrolytes, lipid profile, blood pressure, and heart rate should be monitored. Reference Range The reference range of target serum trough concentrations depends on the time after transplantation. Typically, it is 300ng/mL in the first few weeks, 200ng/ mL over subsequent months, and 100 to 150ng/mL during long-term follow- up. Trough levels should be obtained 12 hours after oral dose (chronic usage), 12 hours after intermittent I. When central venous administration is used, peripheral venipuncture, capillary pin prick, or a double-lumen catheter should be used to draw blood samples for therapeutic drug monitoring. Drug-Drug Interactions Acyclovir, aminoglycosides, diclofenac, amphotericin B, erythromycin, and metoclopramide increase cyclosporine absorption. Ketoconazole, fluconazole, erythromycin, diltiazem, verapamil, and meth- ylprednisolone increase cyclosporine concentration by inhibiting hepatic metabolism. Phenytoin, phenobarbital, carbamazepine, primidone, rifampin, trimetho- prim, and nafcillin decrease cyclosporine concentration by increasing hepatic metabolism. Prednisolone, digoxin, and lovastatin may undergo reduced clearance when used with cyclosporine. Adverse Effects The principal adverse reactions to cyclosporine therapy are renal dysfunction, hypertension, hyperkalemia, tremor, hyperlipidemia, and gingival hyperpla- sia. Forced emesis may be beneficial if performed within 2 hours of ingestion of oral cyclosporine. Tacrolimus Indication Tacrolimus is used as an alternative primary immunosuppressant to cyclosporine in all forms of solid organ transplantation in children. Tac- rolimus seems to be somewhat more potent in preventing acute rejection than cyclosporine. A recent three-arm randomized trial of tacrolimus versus cyclosporine (along with corticosteroids and either sirolimus or mycophe- nolate mofetil as adjunctive therapy) in adult heart transplantation showed lower acute rejection rates in patients treated with tacrolimus. Renal toxicity seems comparable between tacrolimus and cyclosporine in pediatric heart transplantation. Similar to cyclosporine, tacrolimus inhibits T-cell activation by inhibiting cal- cineurin. When renal function is impaired, induction therapy with T-cell-depleting antibodies is generally used with delayed introduc- tion of tacrolimus orally. Pediatric patients clear the drug twice as rapidly as adults, and require higher doses on a milligram per kilogram basis to achieve similar blood concentrations. Tacrolimus is prima- rily eliminated in bile, with less than 1% excreted as unchanged drug in urine. Typical levels are 10 to 15ng/ mL in first few weeks after transplantation, 7 to 10 ng/mL for remainder of first year, and 5 to 7 ng/mL long after transplantation.
Please specify detailed account of sanitation proramme specific to various areas buy doxepin 75 mg line anxiety symptoms twitching, equipment discount 10 mg doxepin anxiety scale 0-5. Whether all the containers of each batch of starting materials is sampled for identification test. How control on temperature and humidity conditions, wherever necessary, maintained in these storage areas. If by electronic data processing system then how access is controlled to enter, modify etc. Whether master formula and detailed operating procedures are maintained as hard copy. Which colour coding system is used to indicate the status of a product and equipment. How returned/unused packaging material like foils is controlled so as to prevent contamination and cross- contamination. Please provide list of reference standard and reference impurities procured from the authentic sources. Please specify the procedures of preparation of working standard from the reference standards. Whether approved specifications for different materials, products, reagents, solvents including test of identity content, purity and quality available. How it is ensured that the sample collected are representative of the whole batch. Whether specification of finished product include (a) the designated name of the product and the code reference; (b) the formula or a reference to the formula and the pharmacopoeial reference; (c) directions for sampling and testing or a reference to procedures; (d) a description of the dosage form and package details; (e) the qualitative and quantitative requirements, with the acceptance limits for release; (f) the storage conditions and precautions, where applicable, and (g) the shelf-life. Whether head of production, quality control and quality assurance unit endorse this documents. Mention shall be made of any substance that may „disappear‟ in the course of processing. Whether the Batch Processing Records for each product on the basis of currently approved master formula is being maintained. Whether periodic audits of distribution center are carried out to access warehousing practices Whether distribution records are part of the batch record. Whether validation studies of processing, testing and cleaning procedures are conducted as per pre defined protocol. How records and conclusion of such validation studies are prepared and maintained. Specify how significant changes to the manufacturing process equipments material etc are controlled. Whether reports of serious drugs reaction with comments and documents immediately sent to Licensing Authority Is there any criteria for action to be taken on the basis of nature of complaint / adverse reaction. If so, by which method: Bubble Point, Diffusive Flow or Pressure Hold Test Sterilization (Autoclaving) 10. Whether the design of 2 closures and containers suitable to make cleaning easy, and to make an air tight seal when fitted to the bottles 11. Whether the material 3 quality of the stoppers and closures ensures that it does not affect the quality of the product and avoids the risk of toxicity 11.
To limit such recurrence generic doxepin 25 mg on-line anxiety care plan, medication should be continued for at least 5–7 days after the lesion appears to be healed 25mg doxepin otc anxiety symptoms 4 dpo. If fluorescein staining does not show reduc- tion of infection in 14 days, it is strongly advised to resort to alternate therapy. Contraindications: Severe bone marrow depression, hypersen- sitivity to ifosamide, pregnancy. Warnings/precautions • Use with caution in patients with the following conditions: kidney or liver disease, compromised bone marrow reserve, prior or con- comitant radiation therapy, prior therapy with cytotropic drugs. Advice to patient • Avoid crowds as well as persons who may have a contagious disease. Editorial comments • This drug should be administered by a physician who is experi- enced in the use of cancer therapeutic drugs. It should be noted that the most effective dose or dose regimen have not been determined. Sit at the edge of the bed for several minutes before standing, and lie down if feeling faint or dizzy. Mechanism of action: Inhibits sodium resorption in distal tubule, resulting in increased urinary excretion of sodium, potasssium, and water. Increase to 5 mg/d if response is not satisfactory after 4 weeks or consider giving another antihypertensive drug. Contraindications: Anuria, hypersensitivity to thiazides or sulfon- amide-derived drugs. Mechanism of action: Inhibits cyclooxygenase, resulting in inhi- bition of synthesis of prostaglandins and other inflammatory mediators. Mechanism of action: Antidiabetic action: Insulin facilitates trans- port of glucose across cell membranes of fat and muscle, resulting in a reduction of blood glucose levels. Hyperkalemia: Insulin causes a shift of potassium from serum into cells, thereby lower- ing serum potassium levels. Increase dose by 2–10 units daily or weekly until satisfactory glucose level is established. Adjustment of dosage • Kidney disease: Creatinine clearance 10–50 mL/min: 75% of normal dose; creatinine clearance <10 mL/min: 25–50% of normal dose. It should be noted that during pregnancy insulin requirements may increase drastically, but then decline imme- diately postpartum. Contraindications: Hypoglycemia, hypersensitivity to specific type of insulin given. Hypo- glycemia may result from decreased food intake, vomiting, excessive exercise, excessive alcohol consumption. If the patient experiences three hypoglycemic reactions in less than 6 months, insulin dosage should be reduced. Patient should be advised how to reverse mild hypo- glycemia: drink fruit juice (orange), eat sugar or candy. Adverse reactions • Common: palpitations, fatigue, subcutaneous lipid, atrophy or hypertrophy, blurred vision. Clinically important drug interactions • Drugs that decrease effects/toxicity of insulin: oral contracep- tives, corticosteroids, dextrothyroxine, diltiazem, epinephrine, smoking, thiazide diuretics, thyroid hormone. Glucose levels of 80–120 mg/dL are considered to be the target for good/optimum con- trol. Monitor blood glucose frequently at times of stress because under such conditions there is an increased need for insulin. A HbA1C value of 6–8% indicates good glucose control whereas values of 11–13% indicate poor glucose control.
SHARE THE DANA LANDSCAPING PAGE